A net gain
Clive’s Corner by Clive Bagshaw
A feature of MicroNews, Clive’s Corner is a place created for the sharing of knowledge, tricks, and tools. The Corner is where you read about clever microscopical hacks - and submit your own. Clive’s Corner is the namesake of SFMS member Clive Bagshaw, who has spent a lifetime looking into microscopes - including 50 years studying protein reactions.
Clive continues to democratize science and save us from ourselves. This is installment #7 of Clive’s Corner… and it’s a catchy one.
The last issue of the Corner involved drilling holes in your microscope and may have had limited appeal. In this one we won’t touch a microscope until the end – it is about catching specimens to observe under a microscope.
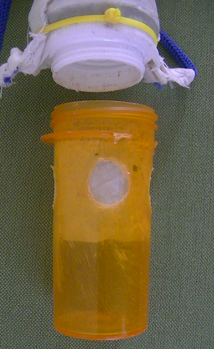
A variety of plankton nets are available on-line with variable specifications. The budget ones cost around $30 to $100 but they have mesh sizes > 100 µm which is fine for larger zooplankton but not most phytoplankton. My interest in getting a small phytoplankton net was sparked by a visit back to my hometown in the UK. I had spent many hours on the seafront there but had never dipped a net in the water for plankton. The nearest item I could find on-line with a 50 µm mesh was a filter bag for cleaning an aquarium for $10.99 (Figure 1a). This was the wrong shape for a plankton net and had no collection bottle at the bottom, but it was a simple matter to cut it into a triangular shape. The leftover mesh was saved for the cod-end as described below. The edges of the net were sealed using iron-on fabric tape, re-enforced by some stitches and a hole in the bottom was left for a snug fit around the cap of a 10 dram pill bottle (Figure 1b).
Figure 1a. A 50 µm mesh filter bag
Figure 1b. The mesh bag modified for plankton collection.
The center of the cap was cut away to allow free flow and the remainder was fixed with a zip tie, with the external thread exposed for connection to the bottle. Four ½ inch (12 mm) holes were drilled near the top of the pill bottle itself and covered with some spare nylon mesh to become the cod-end (Figure 2a). The windows in the cod-end are important as it ensures the water drains through the top of the cod-end rather than at the junction with the net which tends to trap much of the plankton. The cod-end screws into the cap as the original pill bottle intended. Finally, a 50 feet (15 meter) cord was tied to the top of the net and a lead weight added to the bottom. The dimensions of the completed net were 6.5 inches (16.5 cm) top diameter, 15 inches (38 cm) length and an effective cod-end volume of 20 ml below the mesh window.
Figure 2a. Cod end modified from pill bottle.
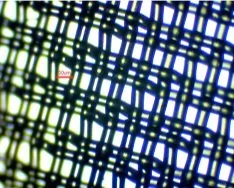
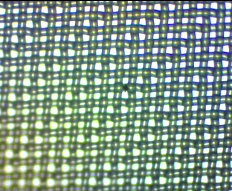
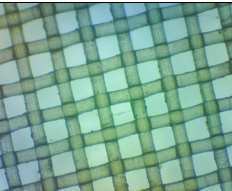
When the net mesh was examined under a stereo microscope, it was evident that the spacing was not that regular with holes around 80 x 50 µm alternating with holes about 20 x 50 µm (Figure 2b). Compare this with the regular mesh of the 20 µm phytoplankton net (Figure 2c) and the 80 µm zooplankton net (Figure 2d) provided by the Plankton Monitoring Network.
Figure 2b. The “50 µm” mesh net
Figure 2c. A 20 µm mesh phytoplankton net
Figure 2d. An 80 µm mesh zooplankton net
In theory, the degree to which a plankton net concentrates the sample can be calculated from the ratio of the volume of the water column sampled to the volume of the cod-end. For a 3 meter tow repeated 5 times the concentration factor comes to a few thousand fold for this net. However, a net provides some back pressure, which can become very significant if the net begins to clog, so some of the water column flows around the net rather than through it. All calculations then go out of the window. This is a particular problem after storms when samples are rich in sediment, or when species like Chaetoceros, with numerous setae, are abundant. The back pressure becomes more significant at higher tow speeds. For a tow of 3 meters depth, it should take at least 4 seconds to raise the net. Further discussions of plankton net design are summarized by Keen (2015). A key equation in these theoretical considerations is the Open Area Ratio, OAR:
OAR= filtering area/mouth area = β*a /A
β = porosity = ratio of the area of the holes to the total area of the mesh.
a = total area of net
A= mouth area
To maximize the OAR, a long net length relative to the diameter of the opening is required (i.e. an angle between the top of the net and its side of >75 degrees). This home-made net had an OAR = 1.3, close to that of the 20 µm full-sized phytoplankton net.
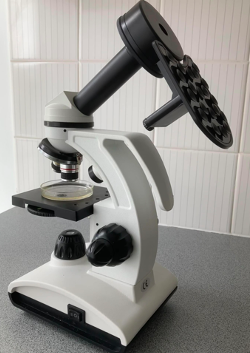
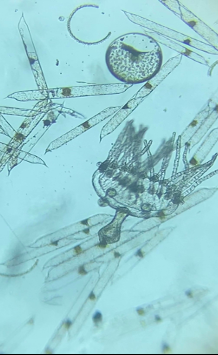
So much for theory, how did it work in practice? With samples collected from the Santa Cruz wharf, the density of plankton in the cod-end was similar to that using the full-sized (meter long) plankton nets. As expected from the mesh size, the ratio of zoo- to phytoplankton was somewhere between that of the 80 and 20 µm nets. During stormy weather, the small home-made net was more manageable as it did not catch the wind as much and the sediment was easier to remove. The 20 ml sample volume was enough to fill 4 Petri dishes, which is plenty for an individual microscopist. But the main reason why I made this net was for traveling. It was easy to pack in the suitcase for my trip back to the UK. The only problem was I didn’t have room for a microscope but managed to find one on-line for £72 ($80; Figure 3a) which was sufficient for monitoring plankton (Figure 3b). What’s more, the microscope came with a battery powered option, so no need to drill any holes in it.
Figure 3a. A portable microscope with a convenient mobile phone holder
Figure 3b. A sample of plankton collected from the Devon, UK coast.