Clive’s Corner #12: DIY Phase Contrast
One of the reasons why plankton are popular subjects for an amateur microscopist is that they are readily observed in a standard brightfield microscope due to the pigments they contain and/or their highly refractive cell walls. However, some objects that a microscopist might encounter are colorless and little detail can be seen because they are only weakly refractive. How can small differences in refraction be enhanced? Take the humble onion skin, a favored test object because thin samples can easily be peeled off by hand to give a single layer of cells. Under brightfield the cell walls are visible but little else within the cell can be seen (Figure 1a). More detail can be seen using phase contrast microscopy (Figure 1b).
a.
b.
Figure 1. A layer of onion skin cells observed under (a) brightfield and (b) phase contrast.
Any material in a specimen that has a different refractive index to its surroundings causes a relative phase shift in the oscillations of the photons. If photons that pass through the material are combined with those that do not, the resultant wave can show constructive or destructive interference which gives the appearance of bright or dark areas in the image (Figure 2). This effect is exploited in Phase contrast microscopy.
Figure 2. (a) Constructive interference of photons which are in-phase gives an enhanced amplitude (intensity) and a bright image, whereas (b) out-of-phase photons result in destructive interference and a dark image.
Generally, two components are required to upgrade a bright field microscope to phase contrast: 1) a phase condenser which generates a cone of light for illumination by means of an annulus at its front focal plane and 2) a phase plate in the objective lens, positioned at its back focal plane. The phase plate contains an etched ring which is slightly thinner (or thicker) than the rest of the plate and is coated to reduce the transmitted light intensity by 70 to 90%. The crucial requirement is that the undeflected cone of light from the condenser, after passing through the slide and objective lens goes directly through the etched ring where it becomes reduced in intensity and phase-shifted (Figure 3a – yellow beam). However, a fraction of the light that goes through the specimen is scattered in different directions and thus avoids the phase ring and continues to the imaging plane where in interacts with the attenuated direct beam (Figure 3a - red beam). Depending on the relative phase shift, these beams will constructively or destructively interfere to give bright or dark features within the image. A fuller description of these principles is available on-line.
Figure 3. (a) The principle of phase contrast using a phase condenser. The sample is illuminated with a cone of light (yellow beam) formed by an annulus in the condenser. Most of this light passes through the specimen and medium and has a high intensity until it passes through the phase plate ring which attenuates the intensity by 70 to 90% (dull yellow beam) and shifts its phase by a ¼ wavelength relative to other positions in the phase plate. Any refractive specimen present on the slide will scatter light (red beam) so that some of it will miss the phase ring but will meet the un-deviated beam at the point of focus. The attenuation of the direct beam ensures it is of comparable intensity to the scattered beam. Depending on the refractive index of the specimen, constructive or destructive interference will occur between the dull yellow and red beams, giving a brighter or darker region in the image compared to the background intensity which passes through the slide. (b) In the modified version of phase contrast employed here, a ring light positioned at a distance, d from the objective lens is used to produce the illumination cone without the need for a condenser.
So much for theory, but how do you adapt a hobby microscope for phase contrast? Phase objective lenses are relatively easy to find second-hand on-line. These lenses come with standard threads and finite or infinite tube length specifications and will fit most microscopes. They can be used as normal bright field objectives because the phase ring occupies a limited area and only attenuates a small fraction of the total light. Phase condensers are also available second hand, but the problem is that they tend to be instrument specific. Not only does the condenser housing have to mechanically fit but each annulus has to match the phase ring in each objective. It is a challenge to make an anulus with the right dimensions manually and center it with micrometer precision, unlike a DIY darkfield stop where a slightly larger than theoretical diameter will work fine. One solution is to abandon the condenser altogether and use a ring light for illumination (Figure 3b) as described by Webb, 2015, Journal of Microscopy, Condenser-free contrast methods for transmitted-light microscopy.
Webb introduced the use of a ring light for phase contrast in conjunction with an inverted microscope for the purpose of gaining a large working distance above the sample. This is useful for investigating unstained live cells where a condenser would block access to micromanipulators. My interest in this approach was sparked by the availability of cheap LED ring lights on-line, which should suffice. These are often marketed as “Angel Eyes” for cars and run on a 12 volt supply. Various diameters are available but those up to around 50 mm are suitable for microscopy. The distance, d, between the objective lens and position of the ring light (Figure 3b) is dependent on the particular phase objective and the ring light diameter. This distance can be determined by shining a flashlight through the objective lens and examining the shadow of the phase ring on a white card beneath (Figure 4a). With an upright microscope, there is limited space beneath the objective which, in the case of my Amcope 120 scope is about 90 mm owing to the in-built illuminator. With a 10x Olympus phase objective this would require a ring light of only 22 mm in diameter. An old Tiyoda microscope with a horseshoe-stand fared better and had 140 mm clearance, which was appropriate for a 40 mm diameter ring with the 10x phase objective (Figure 4b).
a.
b.
Figure 4. (a) Shadow of a 10x Olympus phase objective plate seen at a distance of 90 mm below the lens when illuminated by a flashlight resting on the ocular port. (b) A 40 mm ring light beneath the stage of a Tiyoda microscope matches the required diameter for the 10x phase objective.
When a ring light cannot be accommodated because the required d exceeds the clearance available, a 45o mirror can be mounted in the condenser space and the ring light positioned to the side of the microscope (Figure 5a). In the set-up with the Amscope 120 with a 40 mm ring light, I used a research-grade 45 degree front-faced mirror that I had in my optics collection. However, I also tested an inexpensive 2 x 3 inch reflector from an inspection mirror and found the potential ghost reflection from its glass front face did not cause any significant reduction in image quality. For a given phase objective, the optimal value of d and hence choice of ring light diameter is a compromise. The value of d should be much larger than the focal length of the objective (i.e. >>16 mm for a 10x objective) so that the ring light image is in focus near the back focal plane to coincide with the phase plate. Also, the larger the d, the easier the ring light image is manipulated to achieve superposition with phase plate. However, the intensity of the light falls off as the inverse square of the distance, d. Small diameter LED rings can be made from individual LEDs but additional shielding is required if the width of the LED image exceeds the width of the phase ring. A summary of d values for two phase objectives is given in Table 1. Do not get put off by the numbers - these are just a guide. The final position of the ring light is determined by trial and error, by shifting the ring back and forth and from side to side until its image superposes the phase ring (Figure 5b-d). These movements are of the order of a millimeter or so and do not need precision adjustors, unlike the positioning of the annulus in a phase condenser that requires centering with micrometer precision. This superposition was monitored in the Amscope 120 by removing the microscope eye piece and observing the back focal plane with a DIY phase telescope made from a 5x and inverted 10x eyepiece. The Tiyoda microscope had a built-in Betrand lens in the right-hand eyepiece to achieve the same purpose of viewing the back focal plane.
a.
b.
c.
d.
Figure 5. (a) Positioning of 45-degree mirror beneath the stage of an Amscope microscope and a 40 mm ring light for phase contrast illumination. The ring light was attached to a metal stand by magnets so its position could be easily adjusted. (b) Image of a 10x objective phase ring at the back focal plane. (c) Image of a mis-aligned ring light observed at the back focal plane (d) After manual movement of ring light to superpose its image with the phase ring.
A common test sample for phase contrast are cells obtained by lightly scraping a plastic implement on the inside of the cheek and dabbing this swab, with some saliva, onto the slide and capping with a covers slip. Figure 6 shows the results when using a 10x Olympus A10PL phase objective with a 40 mm LED ring positioned 130 mm from the sample. When the ring light and phase ring were superposed, the image had much more contrast (6b) than with a mis-aligned ring (6a) or a regular (non-phase) objective (6c).
a.
b.
c.
Figure 6, Cheek cell test of phase contrast microscopy (a) Mis-aligned ring light with 10x phase objective, which gives oblique illumination. (b) Aligned ring light with 10x phase objective. (c) Aligned ring with standard 10x objective (i.e. bright field).
There are other methods to improve contrast in brightfield images at zero cost such as closing the condenser aperture to a minimum, which generates Becke lines by light interference (Figure 7a) or blocking the illumination on one side of the condenser to give the pseudo 3D effect of oblique illumination (Figure 7b). These techniques were commonly used before phase contrast was developed in the 1930’s. In addition, digital cameras make it easy to increase the contrast of an image beyond that seen by eye. Darkfield illumination is another technique that emphasizes the scattering properties of an object and can be added to any microscope (Figure 7c). Note that misaligned illumination in phase contrast can give a 3D effect as with oblique illumination (cf. Figures 6a and 7b). This effect can also be produced by blocking half or more of the LED lights on the ring, so that the sample is illuminated by a crescent shape rather than full circle. This technique has been termed Relief Phase Contrast and was used to create the image in Figure 1b.
a.
b.
c.
Figure 7. Alternative methods of enhancing contrast in weakly absorbing samples with a standard (non-phase) objective lens. (a) Standard bright field with an almost closed aperture and contrast enhancement of the digital image. (b) Oblique illumination obtained by blocking the illumination on one side of the light source. (c) Dark field illumination using a patch stop in the condenser filter holder.
With higher power phase objectives, the light from LED ring gave less bright images and above 20x the image brightness was poor. I therefore explored another method to achieve phase contrast described by Rolf Vossen using the built-in microscope illumination. He modified an Abbe condenser by removing the top lens and sticking a circular stop on the remaining lens to match the phase ring inner diameter. An annulus was formed by adjusting the iris below the condenser so its image matched the phase ring outer diameter. Vossen used the field iris on his microscopes (which is lacking on my Amscope 120) but as the top lens of the condenser is absent, and the remaining lens is positioned lower than normal, the original conjugate planes of the microscope no longer hold anyway. As it turns out, the condenser iris can also be used to create a satisfactory annulus. With some initial testing I found that 6 and 12 mm diameter stops were suitable for a 10x Olympus and 40x Zeiss phase objective, respectively. Rather than sticking a stop (cut from black electrical tape) directly on the condenser lens, I used a 30 mm diameter plastic disk, cut from a transparent food container, and stuck a stop in its center. In this way the stop could be rested on the condenser lens (Figure 8a) and easily exchanged or removed for normal viewing. Alignment of the stop, the condenser iris and the phase ring at the back focal plane of the objective was more challenging than aligning the LED rings but it could be done manually. Once aligned, the phase contrast effect was similar to that achieved with the ring lights, but the image with the 40x Zeiss lens was noticeably brighter (Figure 8b).
a.
b.
Figure 8. (a) 12 mm stop centered on the bottom Abbe condenser lens. (b) Phase contrast image of cheek cell using a Zeiss 40X Ph2 objective and 12 mm patch stop on condenser lens using the method of Vossen.
So, what are the final conclusions?
1) An LED ring light is an economic way to generate phase contrast images.
2) In a research setting and with an inverted microscope, ring light illumination provides a large working distance enabling access to the sample for further manipulations.
3) It is easy to manually move the ring light to superpose its image on the phase ring – no precision tools are required.
4) The additional detail observed with an aligned ring light is genuine phase contrast, because contrast is lost when a non-phase objective is used under identical illumination conditions (Figure 6c).
5) Removal of the condenser and lamp lens makes it inconvenient to quickly switch back to a normal brightfield set-up, although the ring light itself was sufficient for brightfield observations with lower power objectives.
6) Modifying the condenser by adding a patch stop is an alternative way to generate a cone of illumination light for phase. It works better at high magnifications than the LED ring light but is more challenging to align.
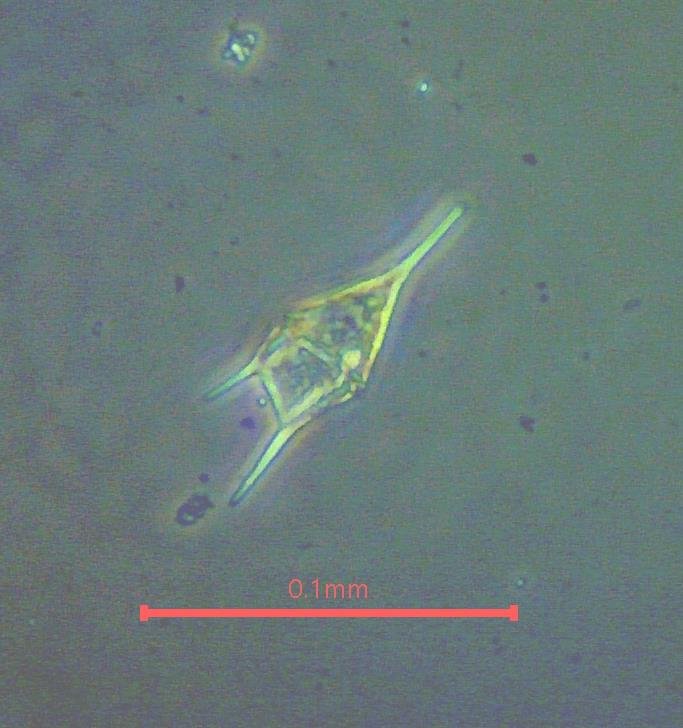
7) Phase contrast works best with thin samples. With thicker samples the halos can become confusing but sometimes they produce appealing images (Figure 9).
a.
b.
Figure 9. Phase contrast images with thick specimens. (a) Tripos (Ceratium) dinoflagellate (b) Cosconidiscus diatom.
I am grateful to Pete Marks for the loan of Olympus 10x and 20x phase objectives for the initial testing used here. As a result of this exercise, I was inspired to search eBay and acquired an Olympus 10x phase objective for $25 in an auction. Thanks also to the San Francisco Microscopical Society for a Zeiss 40x phase contrast objective for testing the procedures.